Quality management system, QMS refers to the management system that commands and controls the organization in terms of quality. Quality management system is a systematic quality control mode established within the organization and necessary to achieve quality objectives. It is a strategic decision of the organization.
It combines resources and processes, and carries out system management with process management method. According to the characteristics of the enterprise, it selects several system elements to combine, which generally includes the process composition related to management activities, resource provision, product realization, measurement, analysis and improvement activities. It can be understood as covering the whole process planning, implementation, monitoring, and The requirements of correction and improvement activities, generally in a documented manner, become the requirements of the organization’s internal quality management work. According to the requirements of the quality management system, the technical committee of quality management and quality assurance of the international organization for standardization has formulated ISO9000 series of standards to apply to organizations of different types, products, scales and properties. On the basis of this standard, different industries have formulated corresponding technical specifications
A newly established company has no formed quality management system that can be used for reference. How to build a new company’s quality system? Here is the experience sharing on how to build the company’s quality system.
(1) As a newly-built company, the leadership decision of the company is the key link to build a quality system, and the top management of the company should make a decision on the implementation and certification of the quality system. From top to bottom.
(2) Prepare the corresponding work plan, promote the establishment of the team, determine the responsibilities, and promote the schedule; Process planning; Formulation of quality policy and quality objectives, etc.
(3) Training for internal auditors and related personnel: managers should know the origin of the standard, master the main content and purpose of the standard, and understand the significance of implementing the standard.
Quality system design and planning
(1) Formulate the current situation of the company’s organizational structure and specify quality policies and objectives.
(2) Identify and determine the relevant processes and quality system terms of the quality system, and prepare the process (requirements matrix) according to the characteristics of products and customer requirements.
(3) Quality responsibility allocation and resource allocation, adjust the organizational structure according to needs, allocate the responsibilities of various quality activities to each functional department, and prepare the function allocation matrix. Identify resource requirements and allocate necessary resources.
(4) Sort out various existing quality related documents and compare them with the system standard terms to determine the list of documents to be prepared;
(5) Make full use of the “PDCA” method to obtain the system documents applicable to the company.
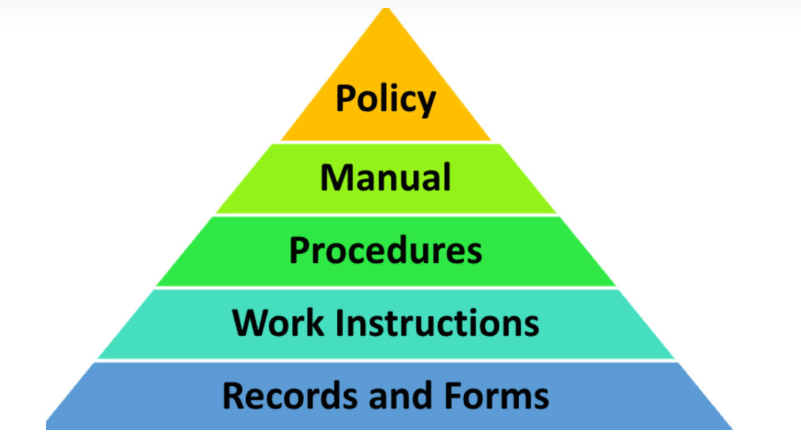
The ISO9001 document system is divided into five levels:
Domestic practice often combines Level 1 and level 2 into level 4: it is not bad to use, which is more convenient for audit.
Level 1 quality policy, level 2 quality manual, level 3 procedure documents,
Level 3 working documents wi, level 4 record forms records forms
Preparation of quality manual:
Determine the applicable quality management system terms according to the standards and in combination with the actual situation of the company; Determine the applicable quality policies, objectives and procedures or prepare corresponding plans; Determine the format and structure of the manual; Classify the existing documents according to the determined format and structure; Complete the draft of the manual; Review and modify to ensure clarity, accuracy, applicability and reasonable structure; Approve, issue, implement, implement the “PDCA” method, and constantly improve it.
Preparation of procedure documents:
Analyze the current documents – sort out and inspect the current enterprise standards, systems, regulations and other documents of the company, and take the requirements of the procedure documents required by the standards as the yardstick to ensure the effective operation of the quality management system;
Prepare the detailed list of procedure documents – according to the quality requirements of the overall design of the quality management system, define the catalogue of procedure documents that should be prepared, determine the procedure documents that need to be newly compiled, improved and improved by comparing with the existing documents, and formulate a plan for gradual preparation; Determine the structure and format of procedure documents; Complete the draft preparation of all procedure documents; Review and modify to ensure clarity, accuracy, applicability and reasonable structure; Approve, issue, implement, implement the “PDCA” method, and constantly improve it.
Preparation of detailed operation documents:
The compilation should adhere to the principle of “the most practical and effective”, and should have good operability; The 5W1H principle is applied to the content, which can be organized in different ways to achieve unique understanding; The specific preparation steps, contents and requirements can refer to the preparation of procedure documents.
Preparation of records:
The design of records shall be synchronized with the preparation of procedure documents / detailed operation documents, and shall be coordinated with them with clear interfaces; Prepare documents of general requirements for records – plan the records required by the system according to the quality manual, procedure documents and quality traceability requirements, and make unified provisions on the identification, cataloging, format, name and content of forms, approval procedures and requirements